Cell Biology of Bacterial Infections

Coxiella burnetii is a Gram-negative, obligate intracellular bacterium responsible for the worldwide neglected zoonosis Q fever, causing severe outbreaks associated with a high economic and health impact. In monocytes, Coxiella adhesion and internalization is mediated by αVβ3 integrins; however, Coxiella also invades a variety of non-phagocytic cells lacking αVβ3 integrins, suggesting that alternative/accessory receptors exist. Moreover, the bacterial adhesins that mediate the interactions with host-cells remain to be identified. Upon entry, Coxiella resides within a tight fitting vacuole positive for typical markers of the early endosomal pathway. The Coxiella-containing vacuoles follow conventional endosomal maturation and the drop in intravacuolar pH is required to activate the secretion of bacterial effectors via a type 4 secretion system (T4SS). Intravacuolar pathogens have evolved a number of strategies to divert the endosomal maturation pathway and avoid fusion with lysosomes. On the contrary, Coxiella has the unique capacity to thrive in a very large parasitophorous vacuole (PV) containing active lysosomal enzymes. Moreover, during infection, Coxiella actively protects infected cells from apoptosis. The late stages of the infectious cycle of Coxiella remain ill-defined and it is unclear how Coxiella infections spread from cell to cell. The obligate intracellular nature of this pathogen had so far hampered its study and consequently, the bacterial factors involved in the intracellular replicative cycle of Coxiella are unknown. The recent development of an axenic culture medium has recently rescued Coxiella from its obligatism and opened the way to the large-scale identification of Coxiella virulence factors.
Our aim is to identify and characterize the
Coxiella
factors involved in the key steps of its intracellular replicative cycle: a) adhesion and entry, b) PV biogenesis, c) cell-to-cell spread and d) protection of the host cell from apoptosis. To this aim, we are generating the first bank of
Coxiella
GFP-tagged mutants by transposon mutagenesis that are being screened using novel and robust imaging-based high content screening (HCS) assays. Our approach has recently led to the identification of
Coxiella
factors possibly involved in bacterial entry, replication and cell-to-cell spread. We are currently conducting a number of studies to validate and characterize the role of these bacterial candidate proteins.
Coxiella
adhesion and entry
: Our HCS assay highlighted
Coxiella
transposon mutants that are affected in host cell internalization. We are particularly interested in transposon insertions in 2
Coxiella
genes that code for transmembrane proteins with protein-protein interaction domains. These specific domains have been reported to play an important role as surface adhesion molecules to a number of host surface receptors. We are currently validating our hypothesis and we will characterize the endocytic pathway that mediates
Coxiella
internalization.
PV biogenesis
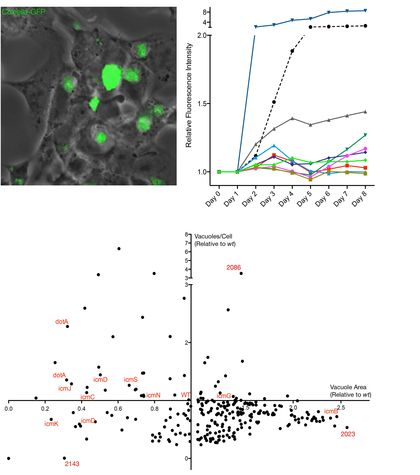
: The
Coxiella
PV is a unique pathogen-induced host cell organelle of remarkable size, composition and plasticity. However, its biogenesis remains largely uncharacterized. The isolation of 18 mutants carrying transposon insertions in genes related to the
Coxiella
T4SS confirmed that secretion of bacterial effectors is required for PV biogenesis. We have also identified a number of
Coxiella
proteins that seem to play an essential role in bacterial replication within infected cells. We are now investigating the possibility that these proteins are T4SS effectors that control the biosynthesis of the PV. To this aim we are conducting a morphometrical analysis of the
Coxiella
PV in different conditions and we are setting up a split-GFP system to investigate bacterial protein secretion and localization during infection.